Named Patient Supply Program India
NAMED PATIENT SUPPLY
Patients who have exhausted their other treatment options and do not meet clinical trial admission requirements have access to named patient supply medications until they are approved by governments around the world. These named patient supply, which is also clustered under the labels of compassionate use, extended access, or named patient supply, are regulated by laws that differ by region, specifying access requirements, data collection, promotion, and drug delivery regulation. Pre-approval demand is typically fulfilled in the United States by therapy INDs (investigational new drug) applications (INDs) or single-patient INDs. These mechanisms, such as extended access services, offer medical access to medications for clusters of people or persons living in the United States. Outside of the United States, named patient services to offer controlled, pre-approval access to medications in response to provider demands on behalf of an individual, or “named,” patients before the medicines are approved in the patient’s home country. Patients can receive medications in late-stage clinical trials or accepted in other countries for a real, unmet medical need by these services before they are authorized in their home country.
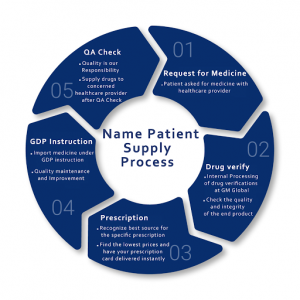
Patient Supply Process
- Patient who has serious problem request for medicine
- Internal processing of drug verifications at we
- Recognizing best source for the specific prescription
- Import medicine under the GDP instruction
- Supply drugs to concerned healthcare provider after QA check
Patients that need a drug that is not readily available in their region can present a challenge for healthcare providers. It’s possible that the medication isn’t legal in their country, that it’s been discontinued, or that it’s just out of stock. Doctors and pharmacists must rapidly identify and dispense patient-specific, difficult-to-find drugs in these cases (often referred to as called patient medicines). We have the expertise and ability to quickly and efficiently procure these named patient medications from all over the world and deliver them to patients in the patient access network who need them. a group of patients
We are well-positioned to serve healthcare providers around the world because we have access to multinational commercial databases and in-house bespoke IT programs, as well as close links to one of the industry’s leading pharmaceutical supply chains. Pharmaceutical firms also contact us asking for an existing organization to coordinate named patient program supply for their drugs because of the international credibility we have gained for Named Patient Medicine supply. Each collaboration is handled on an individual basis, whether it’s enforcing a Managed Patient Access Program or handling cross-border logistics for individual patients.
